People with Medicare Will Face Higher Costs for Some Orphan Drugs Due to Changes in the New Tax and Budget Law | KFF
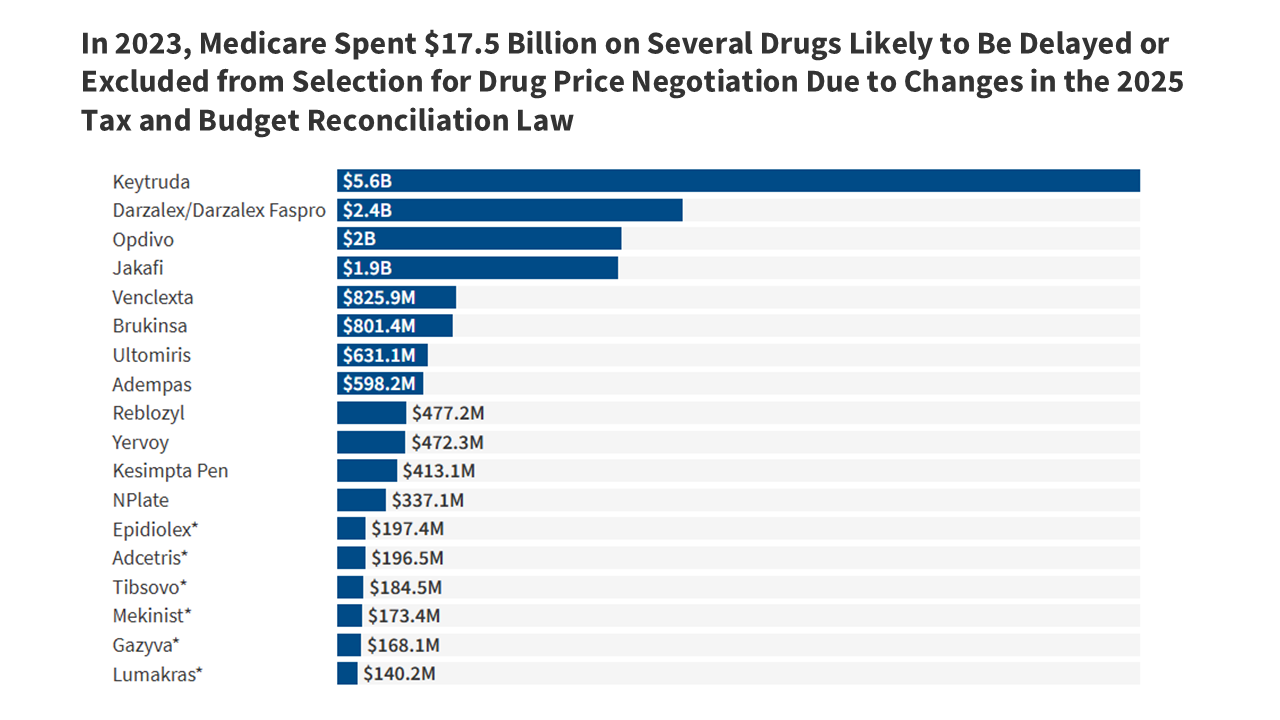
This brief analyzes changes in the new tax and budget reconciliation law that modify which drugs will be selected for Medicare drug price negotiation, which will lead to higher Medicare spending and higher costs for beneficiaries who take these medications.| KFF